天然产物醌类Quinones
Lawsone methyl ether (Synonyms: 2-Methoxy-1,4-naphthoquinone) 纯度: 98.95%
Lawsone methyl ether (2-Methoxy-1,4-naphthoquinone) 是从凤仙花和 Swertia calycina 中分离出的,具有有效的抗真菌和抗菌活性。
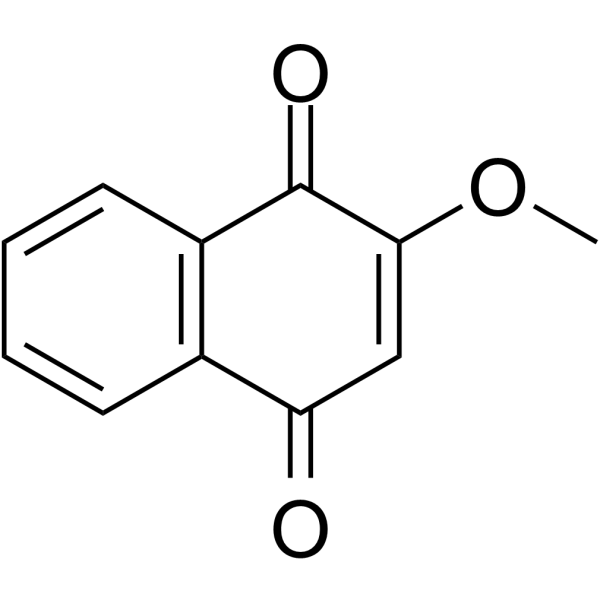
Lawsone methyl ether Chemical Structure
CAS No. : 2348-82-5
| 规格 | 价格 | 是否有货 | 数量 |
|---|---|---|---|
| Free Sample (0.1-0.5 mg) | Apply now | ||
| 25 mg | ¥500 | In-stock | |
| 50 mg | ¥800 | In-stock | |
| 100 mg | ¥1200 | In-stock | |
| 200 mg | 询价 | ||
| 500 mg | 询价 |
* Please select Quantity before adding items.
Lawsone methyl ether 相关产品
•相关化合物库:
- Natural Product Library Plus
- Bioactive Compound Library Plus
- Anti-Infection Compound Library
- Natural Product Library
- Antifungal Compound Library
- Antibacterial Compound Library
- Traditional Chinese Medicine Monomer Library
| 生物活性 |
Lawsone methyl ether (2-Methoxy-1,4-naphthoquinone), isolated from Impatiens balsamina L. and Swertia calycina, exhibits potent antifungal and antibacterial activities[1]. |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 体外研究 (In Vitro) |
The value of both minimal inhibitory concentration and minimal fungicidal concentration of Lawsone methyl ether (2-Methoxy-1,4-naphthoquinone) against Candida was 1.25 lg⁄ml[1]. Shanghai Jinpan Biotech Co Ltd has not independently confirmed the accuracy of these methods. They are for reference only. |
||||||||||||||||
| 分子量 |
188.18 |
||||||||||||||||
| Formula |
C11H8O3 |
||||||||||||||||
| CAS 号 |
2348-82-5 |
||||||||||||||||
| 中文名称 |
二氯甲基甲醚 |
||||||||||||||||
| 运输条件 |
Room temperature in continental US; may vary elsewhere. |
||||||||||||||||
| 储存方式 |
|
||||||||||||||||
| 溶解性数据 |
In Vitro:
DMSO : 50 mg/mL (265.70 mM; Need ultrasonic) 配制储备液
*
请根据产品在不同溶剂中的溶解度选择合适的溶剂配制储备液;一旦配成溶液,请分装保存,避免反复冻融造成的产品失效。 |
||||||||||||||||
| 参考文献 |
|
