天然产物 糖类和糖苷 Saccharides and Glycosides
Glucosamine sulfate;(Synonyms: 硫酸氨基葡萄糖; D-Glucosamine sulfate) 纯度: ge;98.0%
Glucosamine sulfate (D-Glucosamine sulfate) 是一种氨基糖, 是糖基化蛋白和脂质生化合成的突出前体, 用作膳食补充剂。Glucosamine sulfate 也是软骨基质和滑液中糖胺聚糖的天然成分,当外用时,对骨关节炎软骨和软骨细胞具有药理作用。
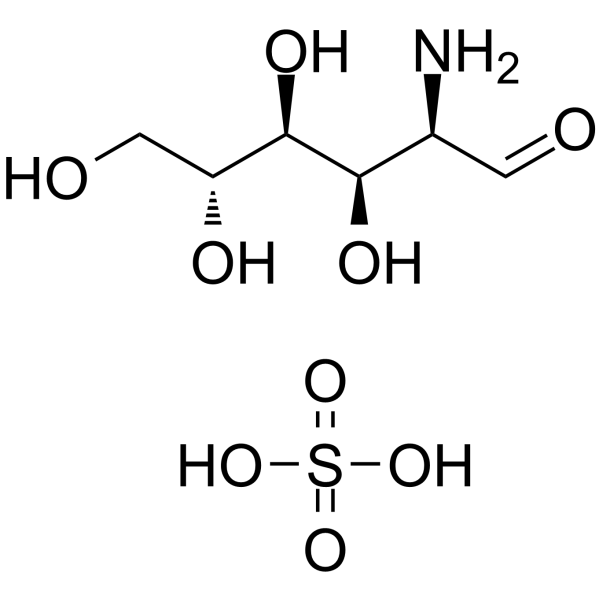
Glucosamine sulfate Chemical Structure
CAS No. : 29031-19-4
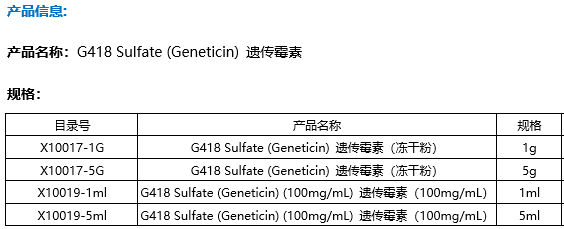
| 规格 | 价格 | 是否有货 | 数量 |
|---|---|---|---|
| Free Sample (0.1-0.5 mg) | ; | Apply now | ; |
| 500 mg | ¥500 | In-stock | |
| 1 g | ; | 询价 | ; |
| 5 g | ; | 询价 | ; |
* Please select Quantity before adding items.
Glucosamine sulfate 相关产品
bull;相关化合物库:
- Natural Product Library Plus
- Drug Repurposing Compound Library Plus
- FDA-Approved Drug Library Plus
- Bioactive Compound Library Plus
| 生物活性 |
Glucosamine sulfate (D-Glucosamine sulfate) is an amino sugar and a prominent precursor in the biochemical synthesis of glycosylated proteins and lipids, is used as a dietary supplement. Glucosamine sulfate also is a natural constituent of glycosaminoglycans in the cartilage matrix and synovial fluid, which when administered exogenously, exerts pharmacological effects on osteoarthritic cartilage and chondrocytes[1]. |
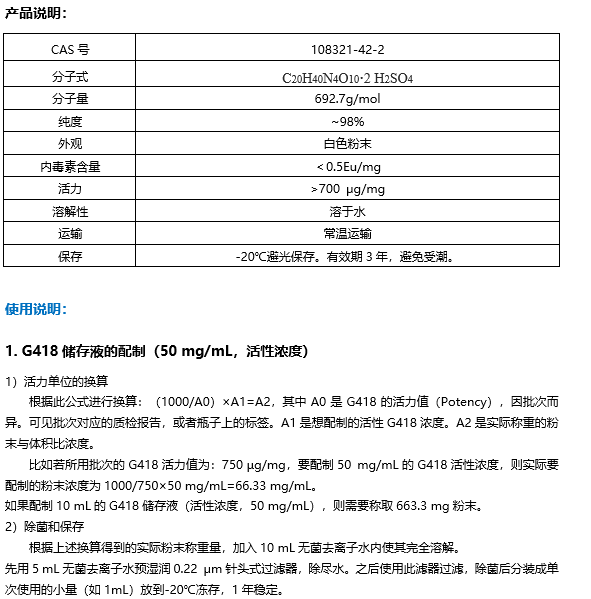
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IC50 Target[1] |
|
||||||||||||||||
| 体外研究 (In Vitro) |
Glucosamine sulfate (D-Glucosamine sulfate) exhibits dose-dependent DPPH antioxidant activity[2]. MCE has not independently confirmed the accuracy of these methods. They are for reference only. |
||||||||||||||||
| Clinical Trial |
|
||||||||||||||||
| 分子量 |
277.25 |
||||||||||||||||
| Formula |
C6H15NO9S |
||||||||||||||||
| CAS 号 |
29031-19-4 |
||||||||||||||||
| 中文名称 |
硫酸氨基葡萄糖;氨基葡萄糖硫酸盐 |
||||||||||||||||
| 运输条件 |
Room temperature in continental US; may vary elsewhere. |
||||||||||||||||
| 储存方式 |
4deg;C, sealed storage, away from moisture *In solvent : -80deg;C, 6 months; -20deg;C, 1 month (sealed storage, away from moisture) |
||||||||||||||||
| 溶解性数据 |
In Vitro:;
H2O : 125 mg/mL (450.86 mM; Need ultrasonic) 配制储备液
*
请根据产品在不同溶剂中的溶解度选择合适的溶剂配制储备液;一旦配成溶液,请分装保存,避免反复冻融造成的产品失效。 |
||||||||||||||||
| 参考文献 |
|