上海金畔生物科技有限公司代理UELANDY荧光染料全系系列产品
Super-n-stain®LS405 Antibody Labeling Kit(LS405抗体标记试剂盒)
产品货号: S601105S/S601105M/S601105L
产品规格: 5-20 μg/kit/20-50 μg/kit/50-100 μg/kit
目录价(元):950/1050/1150
大包装询价
产品概述:
产品内容
| 组分 |
S6011S (5-20 μg) |
S6011M (20-50 μg) |
S6011L (50-100 μg) |
| A: Dye vial |
1 vial |
1 vial |
1 vial |
| B: Super-n-stain® reaction buffer, 10 × |
1 vial of 15 μL |
1 vial of 15 μL |
1 vial of 30 μL |
| C: Super-n-stain® antibody storage |
1 vial of 60 μL |
1 vial of 150 μL |
1 vial of 300 μL |
| D: Ultrafiltration vial (MWCO=10K) |
1 each |
1 each |
1 each |
储存条件
-20ºC 保存,有效期见外包装。
染料信息
|
Super-n-stain® Antibody Labeling Kit
|
货号
|
|
Label Dye
|
Ex (nm)
|
Em (nm)
|
1 × (5-20 μg)
|
1 × (20-50 μg)
|
1 × (50-100 μg)
|
|
Biotin
|
N/A
|
N/A
|
S601101S
|
S601101M
|
S601101L
|
|
FITC
|
494
|
518
|
S601102S
|
S601102M
|
S601102L
|
|
YF®350 SE
|
347
|
448
|
S601103S
|
S601103M
|
S601103L
|
|
YF®405S SE
|
404
|
431
|
S601104S
|
S601104M
|
S601104L
|
|
LS405 SE
|
408
|
452
|
S601105S
|
S601105M
|
S601105L
|
|
YF®488(6)-2 SE
|
490
|
515
|
S601106S
|
S601106M
|
S601106L
|
|
YF®532 SE
|
527
|
558
|
S601107S
|
S601107M
|
S601107L
|
|
YF®555 SE
|
555
|
565
|
S601121S
|
S601121M
|
S601121L
|
|
YF®568 SE
|
562
|
583
|
S601108S
|
S601108M
|
S601108L
|
|
YF®594 SE
|
590
|
617
|
S601109S
|
S601109M
|
S601109L
|
|
YF®647A SE
|
650
|
665
|
S601113S
|
S601113M
|
S601113L
|
|
YF®750 SE
|
750
|
777
|
S601120S
|
S601120M
|
S601120L
|
产品应用
Super-n-stain® 抗体标记试剂盒包含您需要的利用本公司 YF 染料或生物素(Biotin)迅速标记抗体的所有组分。标记过程只需将抗体和染料简单混合于试剂盒内提供的反应缓冲溶液中,进行短时间的孵育即可。Super-n-stain® 染料在标记结束时不再反应,因此无需进一步纯化步骤。标记后,约 4-6 分子染料与 1 分子抗体共价结合。Super-n-stain® 是共价标记,所以用它标记的抗体可以用于多色荧光染色,而不会发生抗体间的染料转移现象。
Super-n-stain® 标记可以兼容 NaN3、低浓度的甘油、Tris 和甘氨酸。试剂盒里提供的微型超滤离心管可以在标记前快速去除不兼容的小分子抗体稳定剂。
标准 Super-n-stain® 标记步骤可以用于多于 IgG (μg) 4 倍的 BSA 或明胶样品。根据您希望标记的 IgG 量来选择试剂盒大小。优化标记步骤适用于稳定蛋白质过剩或腹水的 IgG 样品。在这种情况下,根据需要标记抗体样本的蛋白质总量(IgG + 稳定剂,或腹水中总蛋白量)来选择试剂盒大小。优化步骤也可用于标记 IgG 量低于最低范围的样品,通过添加稳定剂蛋白使总蛋白量达到试剂盒的标记范围。优化步骤不建议用于标记天然抗血清或杂交瘤细胞株培养上清,因为这些样本的抗体含量相对于总蛋白非常低。
当用荧光标记抗体直接进行免疫荧光时,您可能需要使用更高浓度的抗体来实现类似间接免疫荧光法检测(一抗加标记二抗)染色的强度。在我们的内部测试中发现,间接免疫荧光染色结果是直接免疫荧光染色信号的三倍左右。
标记二抗仍可以连接到使用 Super-n-stain® 试剂盒标记的一抗上,因此如果多个来自同一物种的抗体用于多色免疫荧光染色法,二抗无法区分同一物种来源的未标记的一抗和使用 Super-n-stain® 试剂盒标记的一抗。Super-n-stain® 标记抗体可以用作传统间接免疫荧光法检测后的三级染色。UElandy 还提供生物素 Super-n-stain® 标记试剂盒、二次检测使用的 YF 染料标记的链霉亲和素或 YF 染料标记的单克隆生物素抗体。
我们还提供酶标记和荧光蛋白标记的 Super-n-stain® 试剂盒,以及小配体标记试剂盒(请访问 www.uelandy.com)。更多信息,请参见常见问题。
说明书:
 UE-S601105S/S601105M/S601105L
UE-S601105S/S601105M/S601105L
常见问题解答:
◆实验过程中没有纯化步骤,我如何将未标记上的荧光染料除去?
该问题描述了我们研发的关键因素,我们独特的染料、缓冲液组分以及完善的标记策略已经完美地解决了这个问题,具体机制涉及专利保护,不便透露。
◆Super-n-stain® 试剂盒可用于多色免疫标记吗,不同抗体间是否会发生染料转移?
Super-n-stain® 试剂盒抗体与染料是共价结合方式,不存在染料扩散和转移现象。详细信息请参照上面产品说明部分。
◆Super-n-stain® 试剂盒可用于标记抗体以外的蛋白吗?
该试剂盒是针对 IgG 抗体标记优化设计的,不推荐用于标记其它蛋白。Super-n-stain® 标记条件可能会导致 IgM 变性。
◆Super-n-stain® 试剂盒标记一抗和二抗的灵敏性是否一样?
直接免疫荧光检测信号会弱于间接检测,详细信息请参照上面产品说明部分。
◆用标记二抗的间接标记相比,直接标记复合物有哪些优势?
直接免疫染色避免了二抗孵育和清洗的步骤,容许来自同一物种的多个抗体同时使用进行多色检测,可以使用来自同一物种的抗体进行动物组织染色,从而避免了二抗的交叉反应。
◆Super-n-stain® 试剂盒与 Invitrogen’s Zenon 抗体标记试剂盒相比,有哪些优势?
主要优势:
1. YF 染料与抗体共价结合避免了多重染色时染料转移和扩散现象;
2. Super-n-stain® 复合物在储液中可稳定保存几个月之久,而 Zenon 标记物必须在 30min 内使用;
3. Super-n-stain® 复合物体积更小些,因为染料直接与抗体结合,而 Zenon 复合物与抗体片段配合使用;
4. 无需进行 YF 染料与标记蛋白的比例优化,直接混合、染色即可;
5. 无需进行后染固定;
6.没有种属特异性
◆Super-n-stain® 试剂盒与 Innova Bioscience Lightning-link 快速抗体标记试剂盒相比,有哪些优势?
Super-n-stain® 试剂盒使用荧光更亮、光稳定性更好的 YF 染料,试剂盒规格更灵活,可提供至单个抗体标记反应。
◆什么是 YF 染料?
YF 染料是一种水溶性较好、用于标记蛋白和核酸的小分子有机染料。我们可提供多达 20 种的此类 YF 染料。
◆我应如何选择 Super-n-stain® 试剂盒?
每种 YF 染料,我们都提供三种不同抗体标记量的试剂盒,分别是:5-20 μg, 20-50 μg, 50-100 μg。对于没有稳定剂的抗体标记,根据抗体规格,选择其中一种即可,含有稳定剂的抗体标记,请参考产品描述中的表 1。
◆若待标记抗体总量适用于两种 Super-n-stain® 试剂盒,我应如何选择?
例如:标记 50 μg 抗体,我应购买 50-100 μg 抗体标记试剂盒还是 20-50μg 的试剂盒?
遇到这种情况,两种规格都可以得到较好的结果,使用较小规格的效果更好。
◆如何选择染料与蛋白的比例获得较好的标记效果?
如果待标记的抗体量在试剂盒范围内,无需改变染料量或反应时间,因为 Super-n-stain® 试剂盒中染料和反应缓冲液可以确保较好的标记效果。
◆我可以拆分试剂盒组分,以便多次使用吗?
不行,因为 Super-n-stain® 试剂盒已经优化至一个标记单位,我们不建议拆分试剂盒以标记多个抗体或多次使用。
◆抗体浓度是否影响标记效率?
Super-n-stain® 试剂盒较好的标记抗体浓度为 0.5-1 mg/mL,您可通过浓缩或稀释使待标记抗体达到上述浓度范围,小于或超过该浓度范围,会导致标记效率过低或过多。
◆使用标记后的抗体进行免疫荧光染色未观察到荧光信号?
与供应商联系,确保抗体组分、浓度在容许范围内。
◆使用标记后的抗体进行免疫荧光染色未观察到荧光信号怎么办?
与供应商联系,确保抗体组分、浓度在容许范围内。在直接标记前,应确定用于间接标记的一抗的敏感性和特异性。为了得到与间接标记相同的信号强度,直接标记时需要使用更高浓度的一抗,更多信息请参考产品应用部分。共价标记可能会影响某些抗体的反应活性。可以通过实验确定是否是这一因素的原因,即使用 Super-n-stain® 标记的一抗和荧光标记的二抗进行间接免疫染色,以确定 Super-n-stain® 标记后的一抗是否仍具有反应活性。如果有兼容 YF 染料的激发/发射波长的荧光读胶仪或扫描仪,可以通过 SDS-PAGE 电泳确定抗体是否标记上染料,取 0.1-0.5 μg 标记抗体进行变性 SDS-PAGE电泳,然后进行凝胶拍照,你应该可以观察到 IgG 的 55 kDa 的重链和 25 kDa 的轻链对应的两条荧光条带。
使用本产品的文献:
引用文献
1.Paraoxonase 2 decreases renal reactive oxygen species production, lowers blood pressure, and mediates dopamine D2 receptor-induced inhibition of NADPH oxidase
应用方向:polyclonal rabbit anti-PON2 antibody/ polyclonal rabbit anti-D2R antibody
2.The Q loops of the human multidrug resistance transporter ABCB1 are necessary to couple drug binding to the ATP catalytic cycle
应用方向:Primary antibody 4E3
3.Increased Metabolite Levels of Glycolysis and Pentose Phosphate Pathway in Rabbit Atherosclerotic Arteries and Hypoxic Macrophage
应用方向:anti-Ki-67 antibody/anti-macrophage antibody / anti-SMC antibody
4.Mucosal immunoglobulins at respiratory surfaces mark an ancient association that predates the emergence of tetrapods
应用方向:rabbit anti-trout IgT / mouse anti-trout IgM / rabbit anti-Ich
5.Data ofrationalprocessoptimization for the production of a full IgG and its Fab fragment from hybridoma cells
应用方向:IgG
6.Adenoviral L4 33K forms ring-like oligomers and stimulates ATPase activity of IVa2: implications in viral genome packaging
应用方向:polyclonal anti-IVa2 and anti-33K antibodies
7.Principal cell activity induces spine relocation of adult-born interneurons in the olfactory bulb
应用方向:Anti-synaptophysin antibody
8.Enhancing proteasome-inhibitory activity and specificity of bortezomib by CD38 targeted nanoparticles in multiple myeloma
应用方向:anti-CD38 chitosan NPs

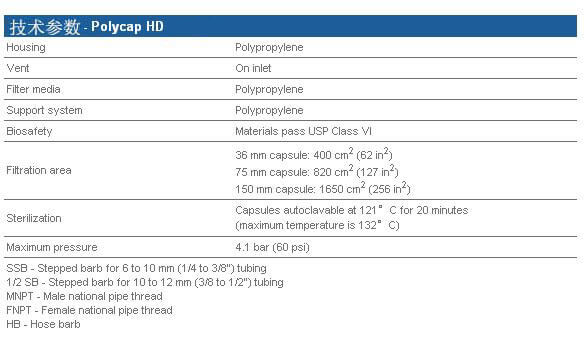
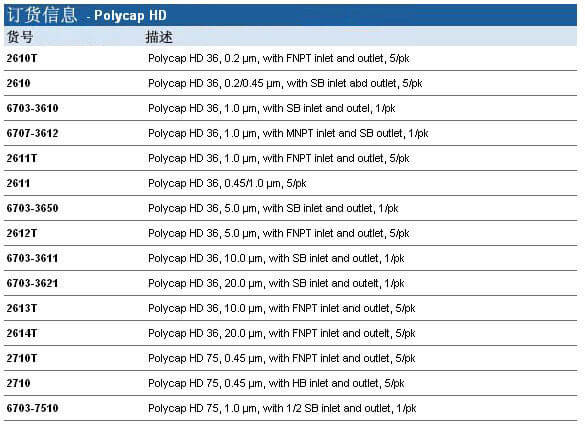
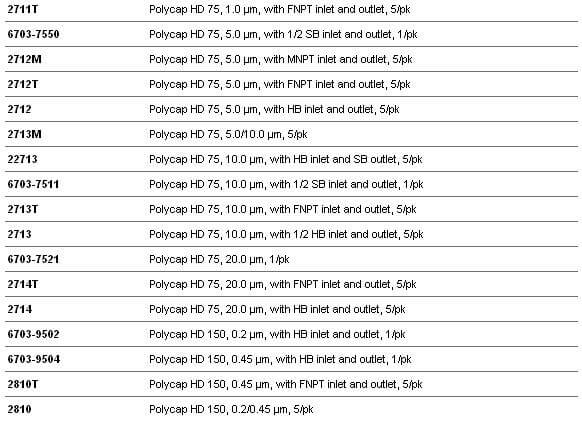
 Whatman Polycap HD(高强度)是一种精心设计的产品,由于它的原料和生产方法使得它有很高的过滤效率和极好的纯度。
Whatman Polycap HD(高强度)是一种精心设计的产品,由于它的原料和生产方法使得它有很高的过滤效率和极好的纯度。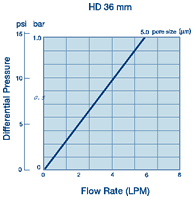 ·100%聚丙烯过滤材质、支持物和外壳,可用于大范围的溶液、pH和温度
·100%聚丙烯过滤材质、支持物和外壳,可用于大范围的溶液、pH和温度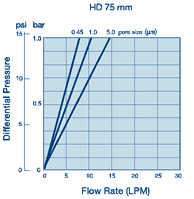 ·化妆品和个人护理用品
·化妆品和个人护理用品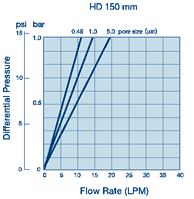




 UE-S601105S/S601105M/S601105L
UE-S601105S/S601105M/S601105L